Surface Energy and Surface Tension
Surface Energy and Surface Tension: Overview
This topic covers concepts, such as, Surface Tension, Intermolecular Forces, Coalescing of Liquid Bubbles & Radius at Common Surface of Coalesced Bubbles etc.
Important Questions on Surface Energy and Surface Tension
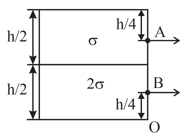
A large tank is filled with two liquids of specific gravities and . Two holes are made on the wall of the tank as shown. Find the ratio of the distances from of the points on the ground where the jets from holes strike.
Water rises to a height of in a capillary tube and mercury falls to a depth of in the same capillary tube. If the density of mercury is and its angle of contact is and density of water is and its angle of contact is , then the ratio of surface tensions of the two liquids is
Two arms of a U-tube have unequal diameters and . If water (surface tension ) is poured into the tube held in the vertical position, find the difference in the level of water in the U-tube. Assume the angle of contact to be zero.
The volume of an air bubble is doubled as it rises from the bottom of a lake to its surface. If the atmospheric pressure is H m of mercury & the density of mercury is n times that of lake water. Find the depth of the lake
The radii of two soap bubbles are and . In isothermal conditions, two meet together in vacuum. Then the radius of the resultant bubble is given by
Pressure inside two soap bubbles are 1.01 and 1.02 atmospheres. Ratio between their volumes is
The excess pressure inside an air bubble of radius r just below the surface of water is . The pressure inside a drop of the same radius just outside the surface is . If T is surface tension then
If the radius of a soap bubble is four times that of another,then the ratio of their excess pressures will be
A film of water is formed between two straight parallel wires of length each separated by . if their separation is increased by while still maintaining their parallelism, how much work will have to be done (surface tension of water )
A drop of mercury of radius 2mm is split into 8 identical droplets. Find the increase in surface energy.( Surface tension of mercury is 0.465 J/m2 )
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
Two small drops of mercury, each of radius R, coalesce to form a single large drop. The ratio of the total surface energies before and after the change is
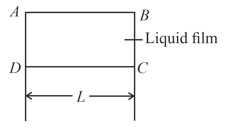
A liquid film is formed over a frame as shown in figure. Wire can slide without friction. The mass to be hung from to keep it in equilibrium is
There is horizontal film of soap solution. On it a thread is placed in the form of a loop. The film is pierced inside the loop and the thread becomes a circular loop of radius . If the surface tension of the loop be , then what will be the tension in the thread
A long wire is placed horizontally on the surface of water and is gently pulled up with a force of to keep the wire in equilibrium. The surface tension in of water is
A thin metal disc of radius floats on water surface and bends the surface downwards along the perimeter making an angle with vertical edge of disc. If the disc displaces a weight of water and surface tension of water is , then the weight of metal disc is
A wooden stick long is floating on the surface of water. The surface tension of water . By putting soap solution on one side of the stick, the surface tension is reduced to . The net force on the stick will be
A bubble has surface tension . The ideal gas inside the bubble has ratio of specific heats . The bubble is exposed to the atmosphere and it always retains its spherical shape. When the atmospheric pressure is , the radius of the bubble is found to be and the temperature of the enclosed gas is . When the atmospheric pressure is , the radius of the bubble and the temperature of the enclosed gas are and , respectively. Which of the following statement(s) is(are) correct?
A water drop of diameter is broken into equal droplets. The surface tension of water is . In this process the gain in surface energy will be
